Water Softeners
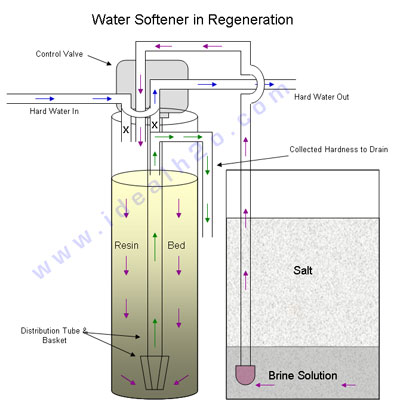
There are two basic functions of a water softener, to collect hardness , and the second is the cleaning process. When the system is collecting hardness it is considered to be the “service run”. The service run allows the water to flow into the machine and down through the resin bed. It is at this time when the water is softened, by ion exchange. The length of the service run depends on the hardness of the water, usage of the water, and the capacity of the system.The following diagram shows a basic path of the water in a softener. First the water travels down through the resin bed, and then at the bottom of the bed it is collected at the basket to travel up the distribution tube and out the fixtures as softened water.
Most softeners today move into the cleaning process or regeneration automatically. This happens by either a predetermined time interval, or based on the usage of the water “Demand Initiated Regeneration”.
During regeneration the collected hardness is flushed out of the system. The length of the regeneration is determined by the flow rate of the water and the size of the system. The following diagram shows a basic path of the water during the regeneration. The first step for a water softener going into regeneration bypasses the internal flows of water. This allows hard water to continue flowing to the building while the system cleans itself. Next the system goes in to backwash to loosen the resin bed, and send collected particulate matter to drain. The softener then draws brine solution from the salt tank. The introduction of brine initiates the ion exchange. After a period of time the residual brine that is left behind is rinsed free from the resin bed and the softener is ready for another service run.

Reverse Osmosis (RO)
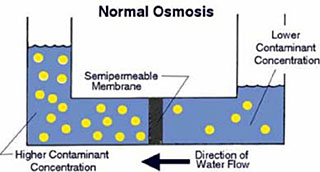
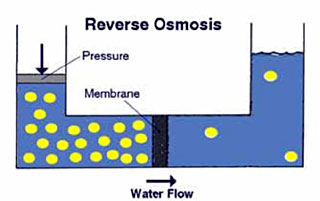
A semi-permeable membrane , like the membrane of a cell wall or a bladder, is selective about what it allows to pass through, and what it prevents from passing. These membranes in general pass water very easily because of its small molecular size; but also prevent many other contaminants from passing by trapping them. Water will typically be present on both sides of the membrane, with each side having a different concentration of dissolved minerals. Since the water in the less concentrated solution seeks to dilute the more concentrated solution, water will pass through the membrane from the lower concentration side to the greater concentration side. Eventually, osmotic pressure (seen in the diagram below as the pressure created by the difference in water levels) will counter the diffusion process exactly, and equilibrium will occur.The process of reverse osmosis forces water with a greater concentration of contaminants (the source water) into a tank containing water with an extremely low concentration of contaminants (the processed water). High water pressure on the source side is used to "reverse" the natural osmotic process, with the semi-permeable membrane still permitting the passage of water while rejecting most of the other contaminants. The specific process through which this occurs is called ion exclusion, in which a concentration of ions at the membrane surface from a barrier that allows other water molecules to pass through while excluding other substances.
Semi-permeable membranes have come a long way from the natural pig bladders used in the earlier osmosis experiments. Before the 1960's, these membranes were too inefficient, expensive, and unreliable for practical applications outside the laboratory. Modern advances in synthetic materials have generally solved these problems, allowing membranes to become highly efficient at rejecting contaminants, and making them tough enough to withstand the greater pressures necessary for efficient operation. Even with these advances, the "reject" water on the source side of a Reverse Osmosis (RO) system must be periodically flushed in order to keep it from becoming so concentrated that it forms a scale on the membrane itself. RO systems also typically require a carbon prefilter for the reduction of chlorine, which can damage an RO membrane; and a sediment prefilter is always required to ensure that fine suspended materials in the source water do not permanently clog the membrane. Hardness reduction, either through the use of water softening for residential units or chemical softening for industrial use, may also be desirable in hard water areas.
Low Pressure (Residential) Systems
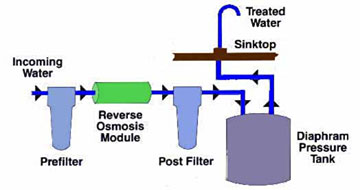
Low pressure RO systems generally refer to those systems with water feed pressure of less than 100 psig. These are the typical countertop or under sink residential systems that rely primarily on the natural water pressure to make the reverse osmosis process function; a typical system is shown schematically below.Countertop units typically have a non-pressurized storage tank; Under the sink units typically have a pressurized accumulator storage tank where the water pressure tends to increase as the tank fills. This pressurized system provides sufficient pressure to move the water from the under sink storage tank to the faucet. Unfortunately, this also creates a back pressure against the membrane, which can decrease its efficiency. Some units overcome this by using non-pressurized tanks with a pump to get the treated water where it is needed.
Low pressure units typically provide between 2 and 15 gallons per day of water, with an efficiency of 2-4 gallons of reject water per gallon of treated water. Water purity can be as high as 95 percent. These systems can be highly affordable, with countertop units starting at about US $350, and under sink units starting at about US $675. These units produce water for a cost as low as ten cents per gallon once maintenance and water costs are factored in. Maintenance usually requires replacing any pre- or post-filters (typically one to four times per year); and the reverse osmosis cartridge once every two to three years, depending on usage.
What Reverse Osmosis Treats
Reverse osmosis can treat for a wide variety of health and aesthetic contaminants. Effectively designed, RO equipment can treat for a wide variety of aesthetic contaminants that cause unpleasant taste, color, and odor problems like a salty or soda taste caused by chlorides or sulfates. RO can also be effective for treating health contaminants like arsenic, asbestos, atrazine (herbicides/pesticides), fluoride, lead, mercury, nitrate, and radium. When using appropriate carbon pre-filtering (commonly included with most RO systems), additional treatment can also be provided for such "volatile" contaminants as benzene, trichloroethylene, trihalomethanes, and radon. Some RO equipment is also capable of treating for biological contaminants like Cryptosporidium. The Water Quality Association (WQA) cautions, however, that while RO membranes typically remove virtually all known microorganisms and most other health contaminants, design considerations may prevent a unit from offering foolproof protection when incorporated into a consumer drinking water system. When looking for a product to treat for a given health contaminant, care should be used to find products that have been tested successfully for such purposes at a quality testing laboratory.This article first appeared in the Water Review Technical Brief, (1995) Volume 10, No. 3; a publication of the Water Quality Research Council; Copyright 1995 by the WQA.
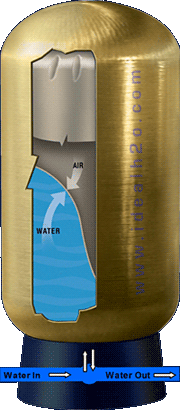
Well Tank (Bladder Style)
The most commonly used type of tank today; the captive air well tank is an efficient way of delivering consistent pressure to a building which requires little or no maintenance.The bladder style tank utilizes a large pressurized balloon (bladder) to create stored energy which when released, will force the water from the tank and out to the fixture or faucet. When the pressure reaches the “cut in” point on the switch; the pump is switched on and the water is forced up from the well and into the tank where it collapses the bladder, compressing the air inside. The pump will continue to pump water into the tank until the “cut off” pressure is reached. At this time the bladder is compressed to an equal pressure to that of the “cut out” pressure. When a faucet is opened the bladder pushes down on the water, forcing it out of the tank and up to the faucet.


